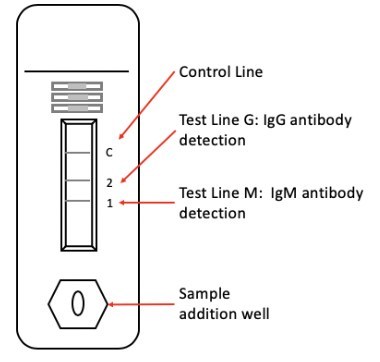
ZEUS's lateral flow test uses patient serum, plasma or whole blood and provides results in 15 minutes. Assay performance has been confirmed by testing sample cohorts from healthy donors, confirmed positive COVID-19 patients and cross-reactive samples acquired from heavily afflicted regions in both China and the United States. The request for EUA is under review by the FDA and if received ZEUS expects to have the test available to ship to customers in early May 2020.
Rapid detection of cases and contacts, appropriate clinical management, infection control, and community mitigation efforts are critical to respond effectively to the COVID-19 pandemic. Results from the ZEUS test will help address the urgent health concerns surrounding the public health threat posed by COVID-19. Identifying individuals with specific antibodies to the SARS-CoV-2 virus provides valuable diagnostic information relating to previous exposure to the SARS-CoV-2 virus.
ZEUS Scientific is a trusted partner with over 40 years of in vitro diagnostic experience providing high-quality diagnostic solutions. Our New Jersey-based, FDA-inspected manufacturing facility is certified and audited to ISO 13485 (2016), FDA Quality System Regulations (1996: 21 CFR § 820) and IVD 98/79/EEC. In these difficult times you can count on ZEUS to apply our expertise and assist in the fight against COVID-19.
In addition to this rapid in vitro diagnostic test, ZEUS is also pleased to announce development of a corresponding ELISA test system, also for detection of antibodies to SARS-CoV-2. Further updates in conjunction with this higher throughput, automatable option will be provided in the near future.
Aware of her elements, Neha writes the best articles across industries including electronics & semiconductors, automotive & transportation and food & beverages. Being from the finance background she has the ability to understand the dynamics of every industry and analyze the news updates to form insightful articles. Neha is an energetic person interested in music, travel, and entertainment. Since past 5 years, she written extensively on sectors like technology, finance and healthcare.

Smarter Decisions with Smart News
Smart Market News is committed to getting its readers the latest updates and insights on industries that help in making “smarter” business decisions. With insights and inputs from corporate decision makers, we bring you the stories of adopting innovative solutions and strategies that have been changing the world. Our editorial insights on products, solutions, companies, and adoption of best practices not only help in understanding the markets better, but also prove to be a complete package for your information needs.